Valence Bond Theory
Valence Bond Theory: Overview
This topic covers concepts such as Valence Bond Theory, Comparison of Sigma and Pi Bonds, Lateral Overlapping, Axial Overlapping, Formation of H2 Molecule According to Valence Bond Theory, Directional properties of Bonds, etc.
Important Questions on Valence Bond Theory
In which of the following molecules both phenyl rings are not coplanar ?
Which of the following plot represents potential energy of a pair of nuclei as a function of their separation:
Which one of the following does not have hybridised carbon ?
Number of sigma bonds in is :
The correct statement for the structure of is
The number of sigma and pi bonds in ethyne molecule are respectively,
Which among the following represents zero overlap?
Which of the following has bonding?
Propyne molecule contains sigma and _____ pi bonds.
The sum of number of sigma and pi bonds formed between two carbon atoms in are:
Explain why covalent bonds are directional in nature ?
Explain the directional properties of bonds ?
Number and type of bonds between two carbon atoms in CaC2 are
Which of the following is not tetrahedral in shape?
The number of and bonds in allyl isocyanide are
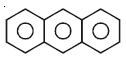
The total number of sigma and pi bonds present in the given anthracene molecule are:
How does oxygen molecule form?
Among the following statements which is false?
bonding is not present in the molecule :
Assertion Six unpaired electrons present in gaseous Cr atoms.
Reason Half filled orbital has greater stability.
